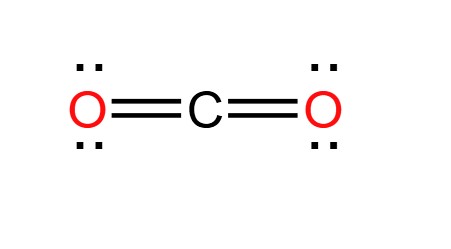
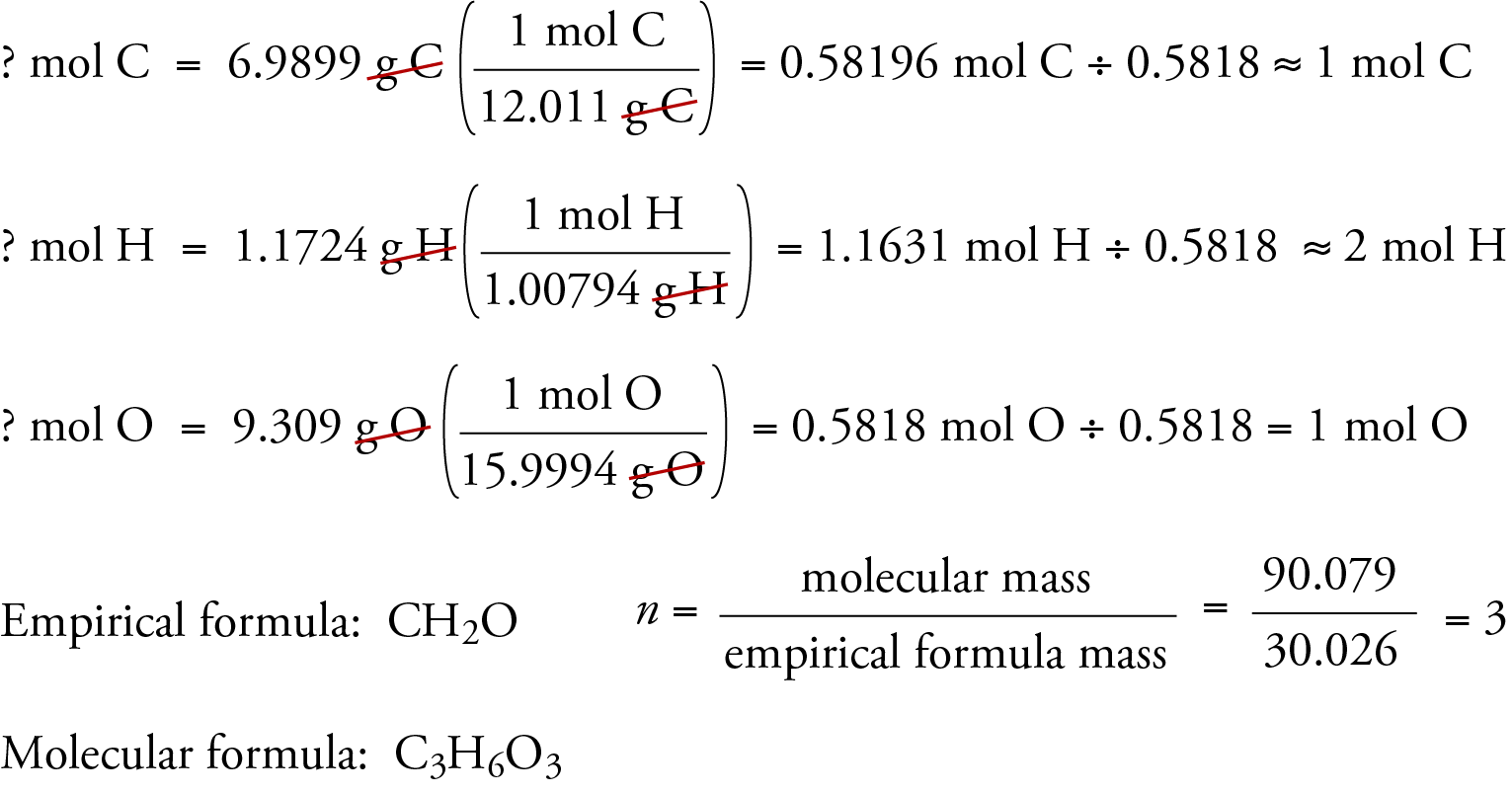
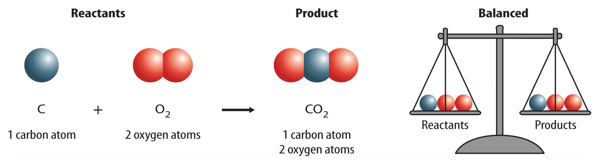
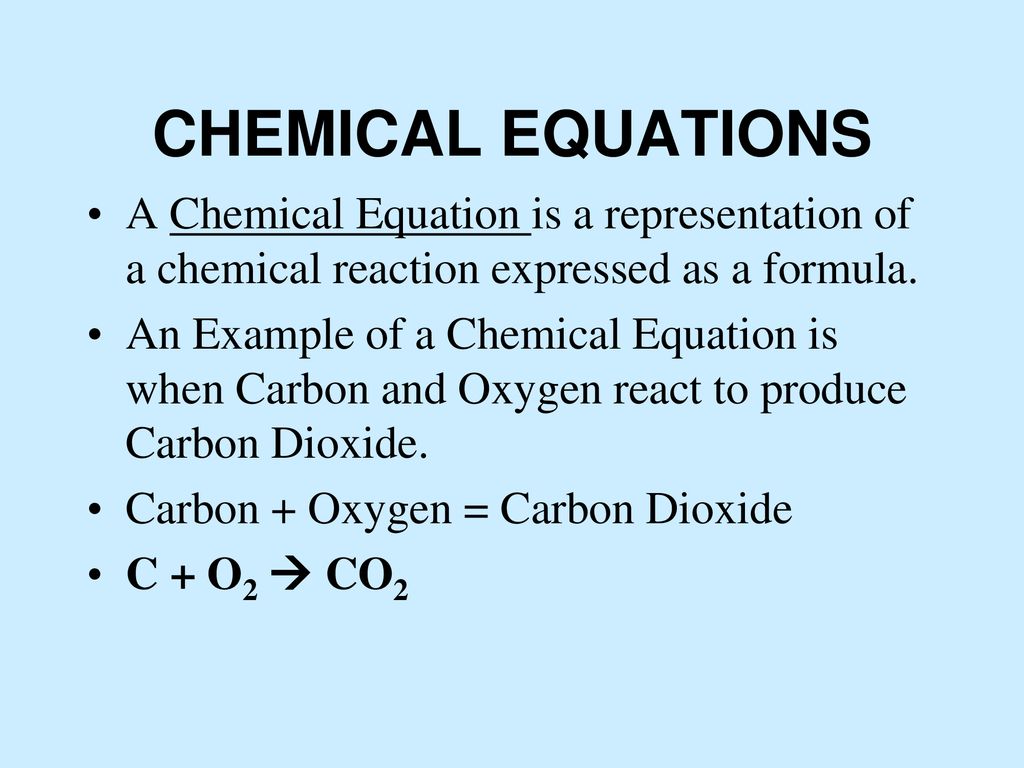
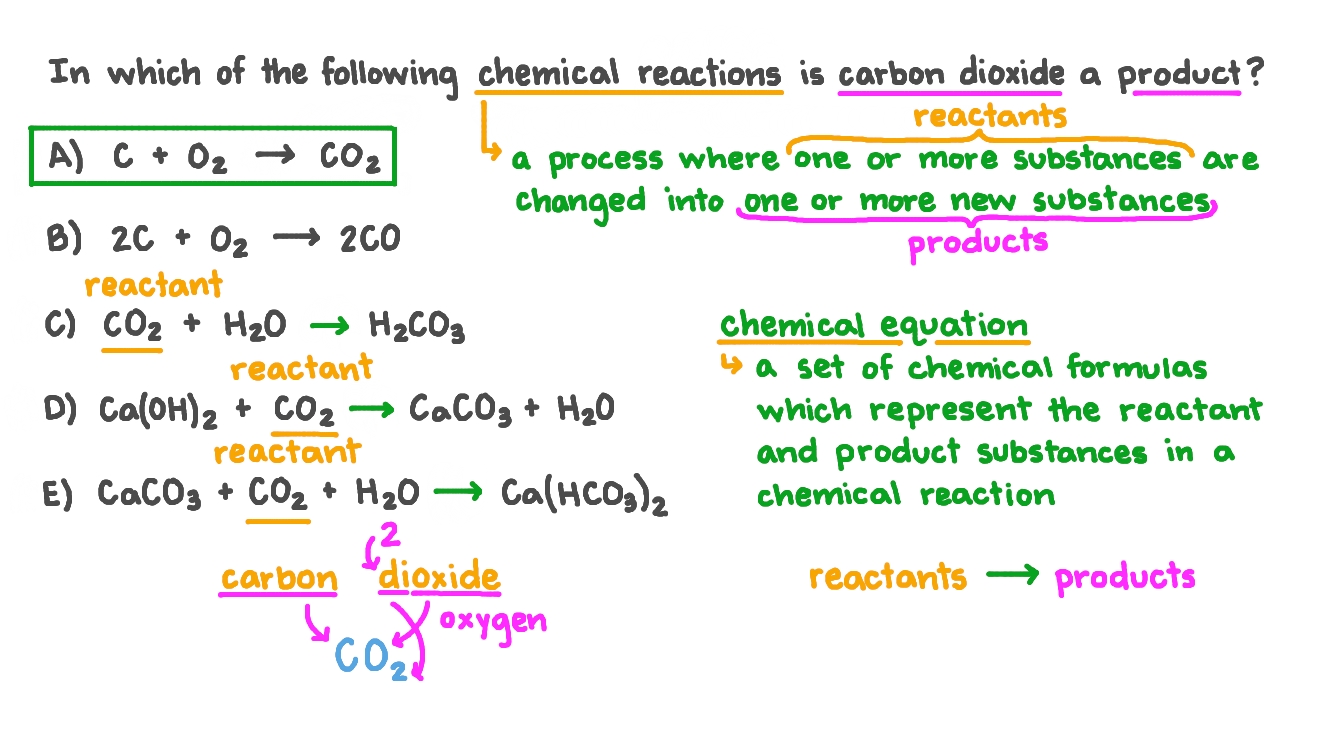
The valency of carbon is 4 and that of oxygen is 2 . What is the molecular formula of carbon dioxide?
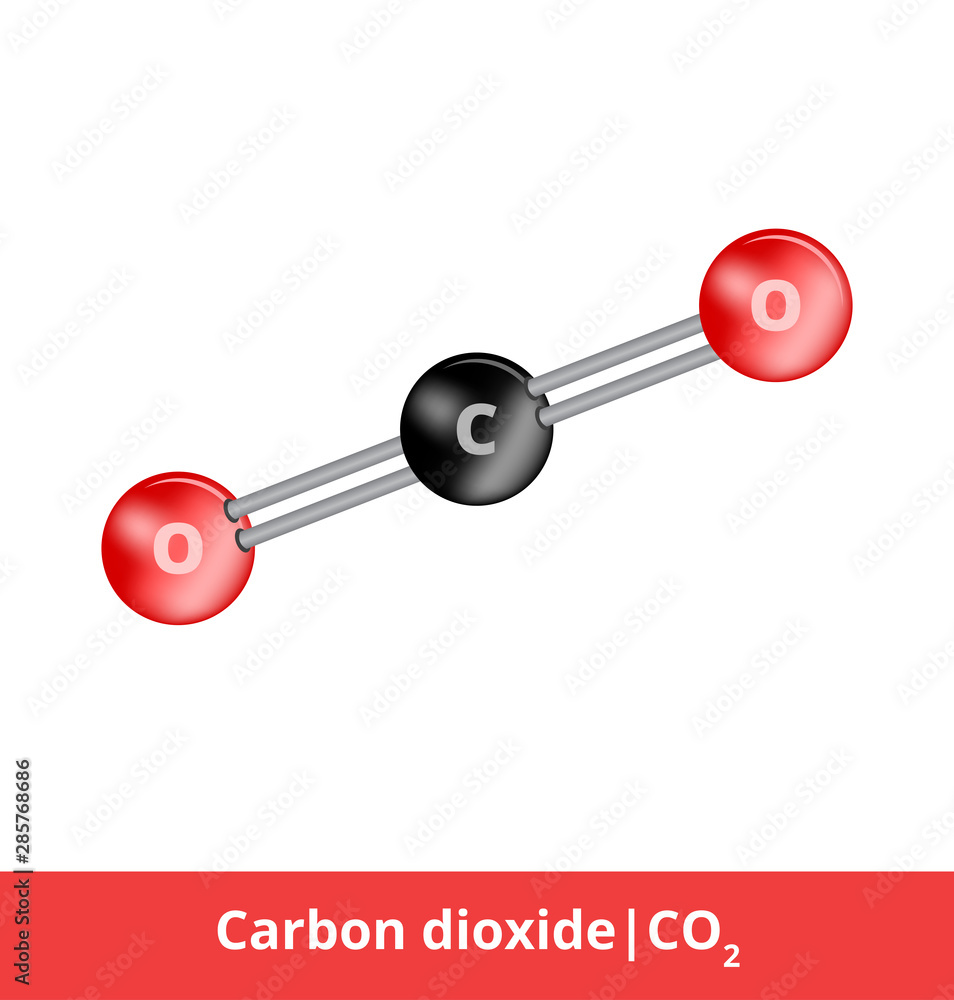
Vector ball-and-stick model model of chemical substance. Icon of carbon dioxide molecule CO2 consisting of carbon and oxygen. Structural formula suitable for education isolated on a white background. Stock Vector | Adobe
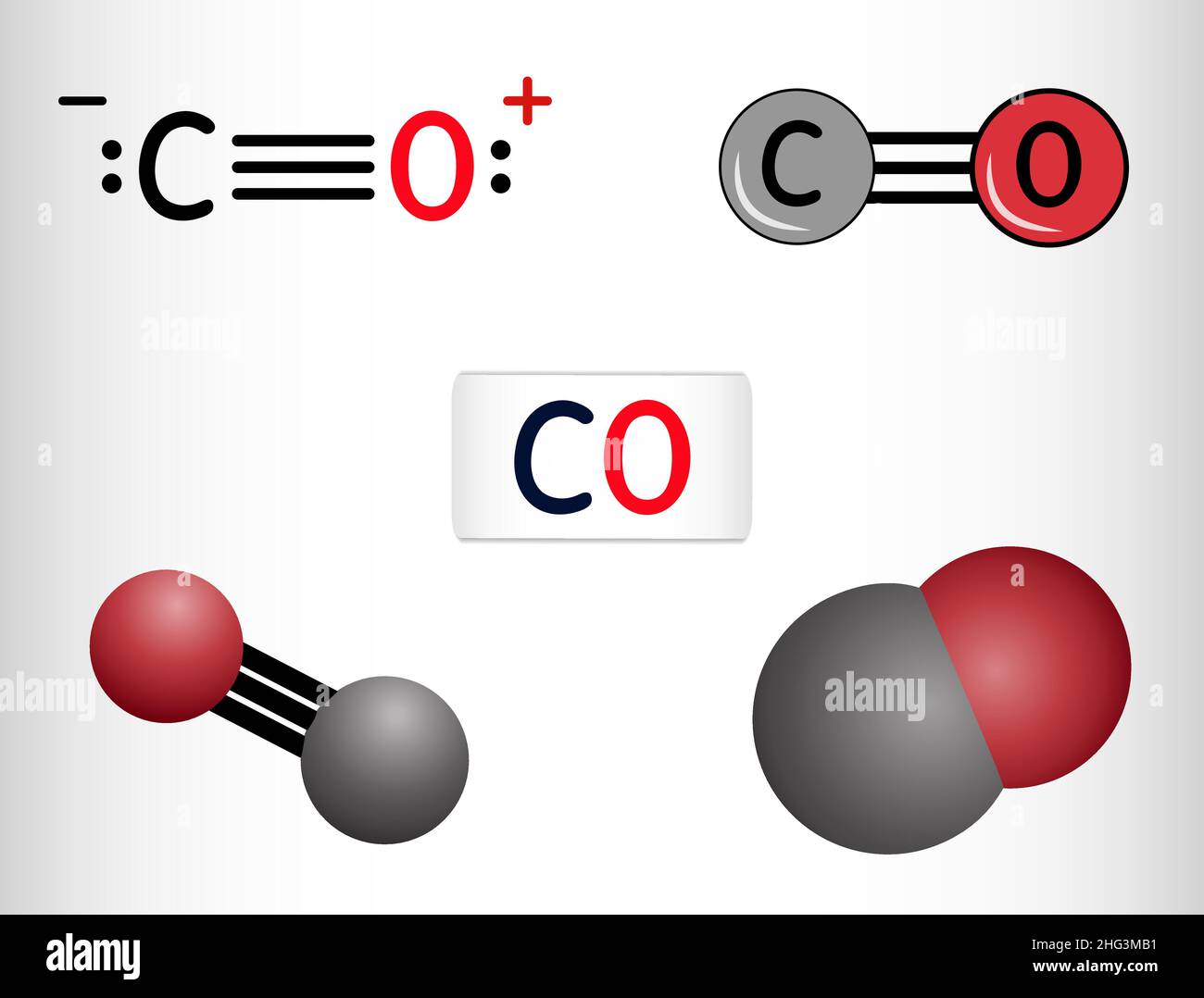
Carbon monoxide, CO molecule. Сarbon and oxygen atoms are connected by a triple bond. Structural chemical formula and molecule model. Vector illustrat Stock Vector Image & Art - Alamy
Carbon has 4 valency and Oxygen has 2 Valency. When these both combine, they should form C2O4, but it forms CO2, why? - lo6klogg