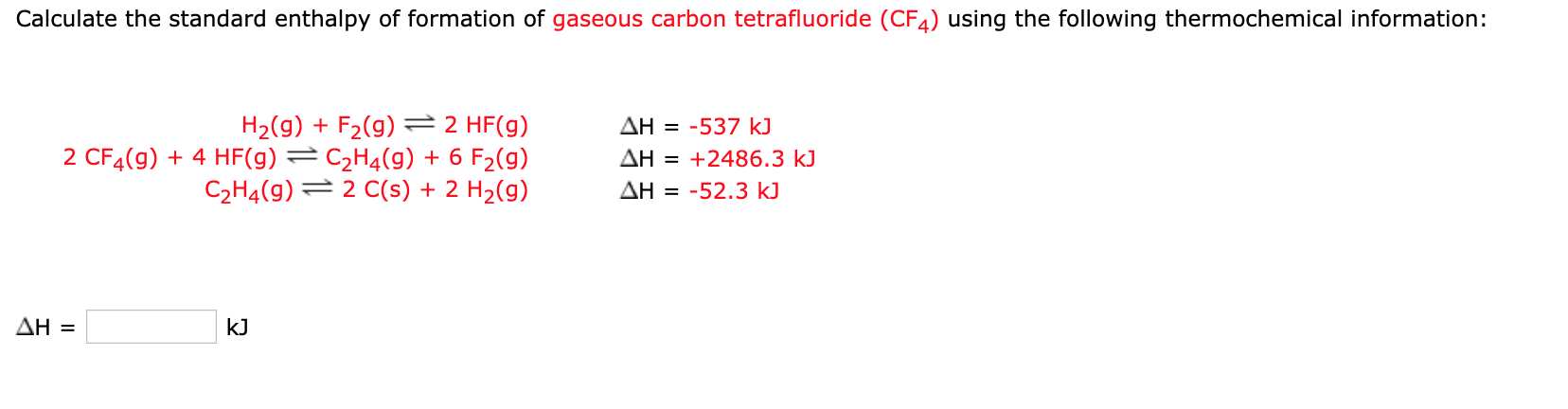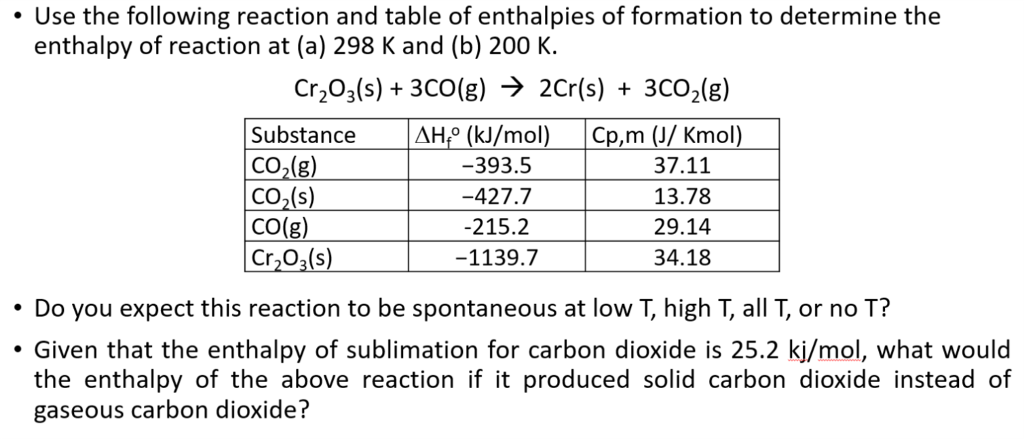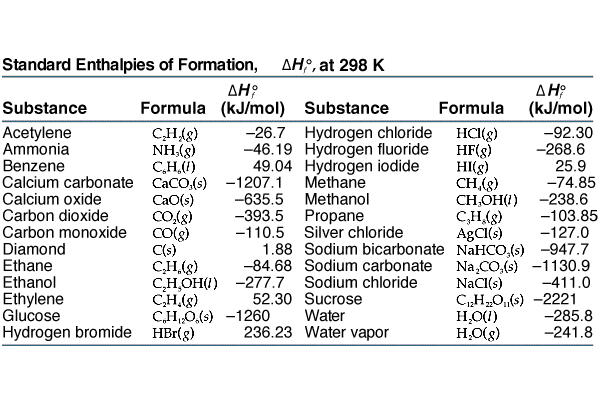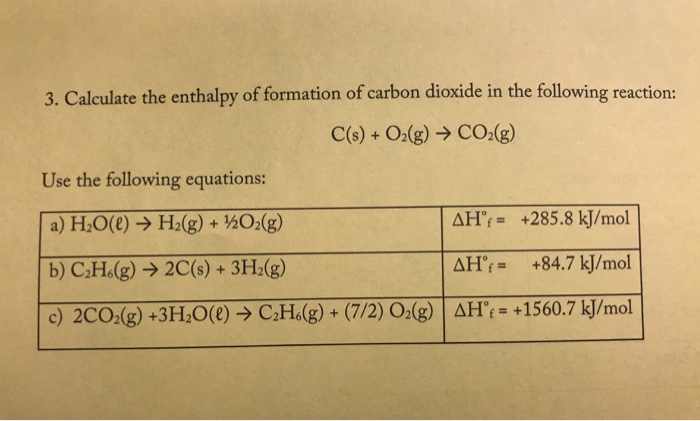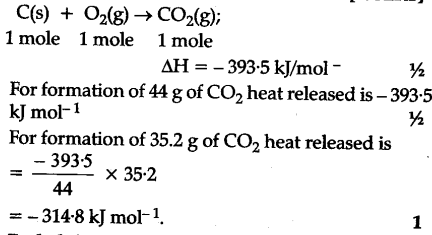
Enthalpy of combustion of carbon to C${{O}_{2}}$ is -393.5 kj/mol. Calculate heat released upon formation of 35.2g of C${{O}_{2}}$ from carbon and ${{O}_{2}}$ gas? - CBSE Class 11 Chemistry - Learn CBSE
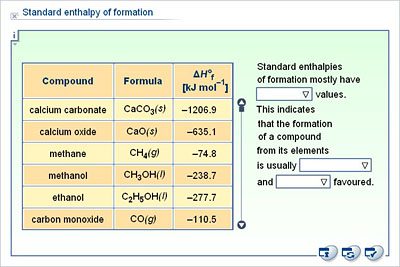
![PDF] Prediction of Enthalpy of Formation in the Solid State (at 298.15 K) using Second-Order Group Contributions. Part 1. Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds | Semantic Scholar PDF] Prediction of Enthalpy of Formation in the Solid State (at 298.15 K) using Second-Order Group Contributions. Part 1. Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/c766ff3889423f02f154fa43603214c3fec1f15d/4-Table1-1.png)
PDF] Prediction of Enthalpy of Formation in the Solid State (at 298.15 K) using Second-Order Group Contributions. Part 1. Carbon-Hydrogen and Carbon-Hydrogen-Oxygen Compounds | Semantic Scholar

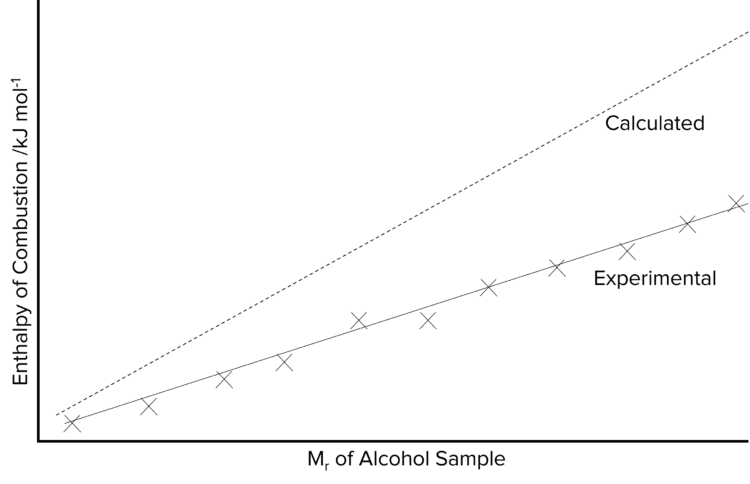
Enthalpy of combustion of alcohols data trend graph explaining trend pattern determining delta H combustion comparison with ethers equations advanced A level organic chemistry revision notes doc brown

Why does the enthalpy of combustion for alkanes increase, e.g. from methane to propane, etc.? - Quora
66 Enthalpies of formation of co(g),CO2(g),N2O(g)and N2O4(g) are 110, 398, 81, 97 KJ/lol respectively. Find value of H for the reaction. N2O4(g) +3CO(g) — >N20(g)+3CO2(g)

The enthalpies of formation of carbon nanomaterials as a key factor for understanding their structural features - Physical Chemistry Chemical Physics (RSC Publishing)
62.The Enthalpies of combustion of carbon and carbon monoxide are 390 kJ and 278kJ respectively. The enthalpy of formation of carbon monooxide is? a) 669 kJ b) 112 kJ c) 112 kJ d) 668 kJ