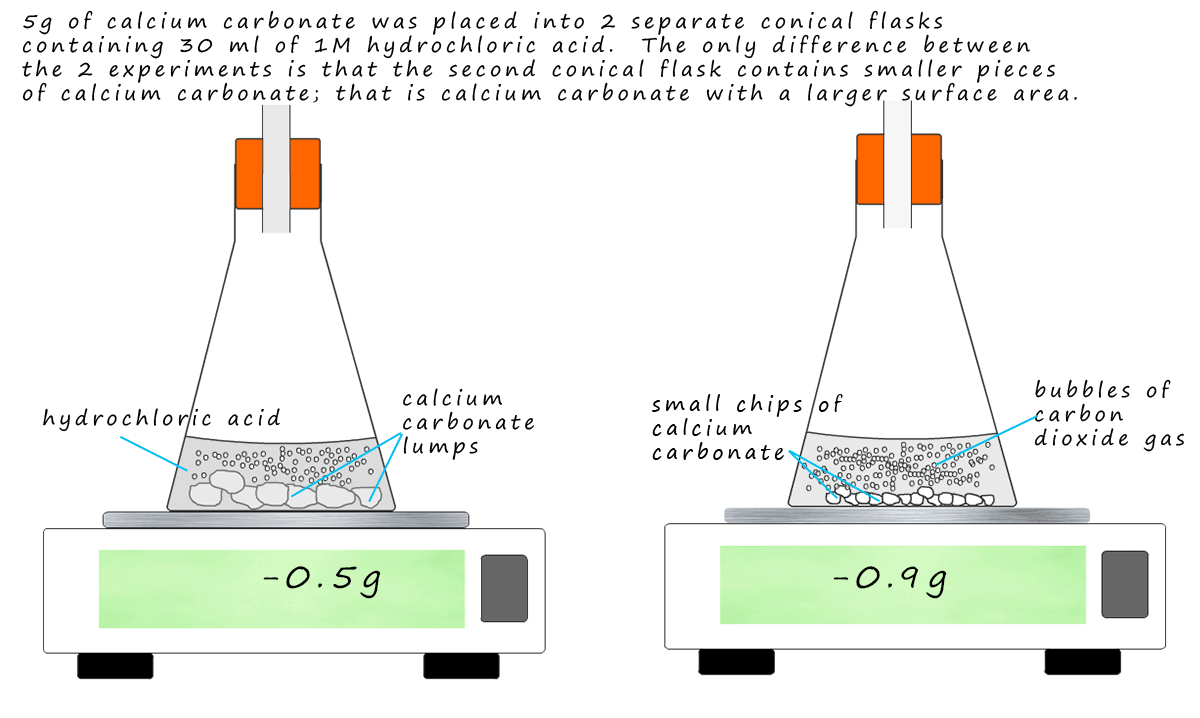
Bildagentur | mauritius images | Reaction rates. Reaction rate increases with concentration of reactants. This effect is demonstrated here using the reaction of chalk (calcium carbonate, CaCO3) with hydrochloric acid (HCl). Carbon
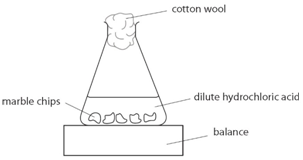
3:15 practical: investigate the effect of changing the surface area of marble chips and of changing the concentration of hydrochloric acid on the rate of reaction between marble chips and dilute hydrochloric

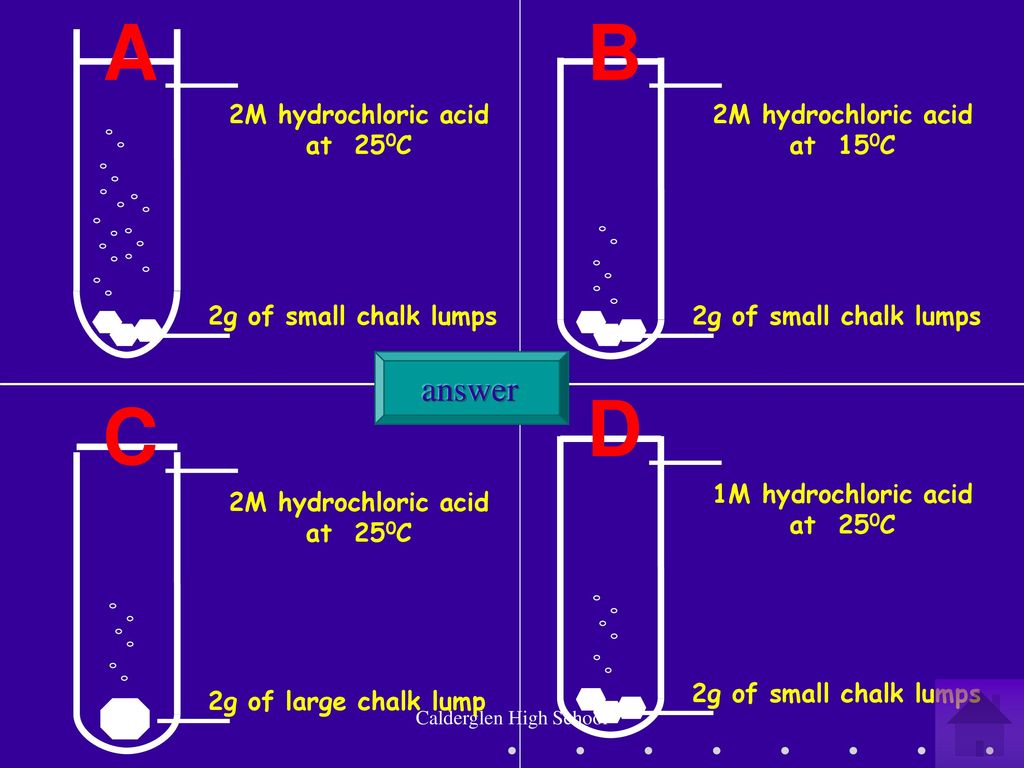
SOLVED: When calcium carbonate reacts with hydrochloric acid, a surprising reaction occurs: CaCO3(s) + 2HCl(aq) -> H2O(l) + CaCl2(aq) (a) Balance the chemical equation above. (b) If a 2.82-g stick of chalk

Reaction of chalk and acid. This is an example of an acid-carbonate reaction. Chalk contains calcium carbonate, and the acid here is hydrochloric acid Stock Photo - Alamy
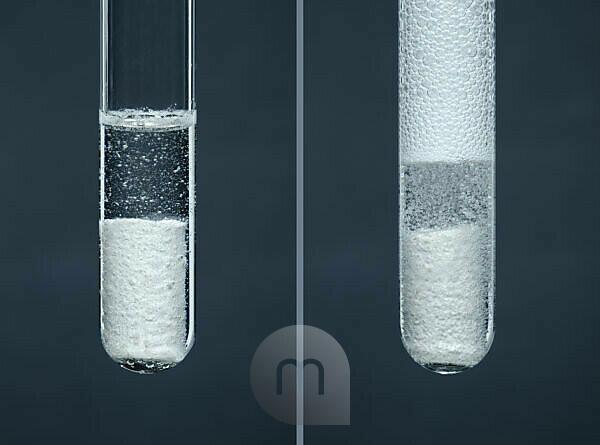
Bildagentur | mauritius images | Rate of reaction. Reaction of chalk with hydrochloric acid is used to demonstrate that the rate of reaction depends on concentration. Chalk is calcium carbonate, it reacts