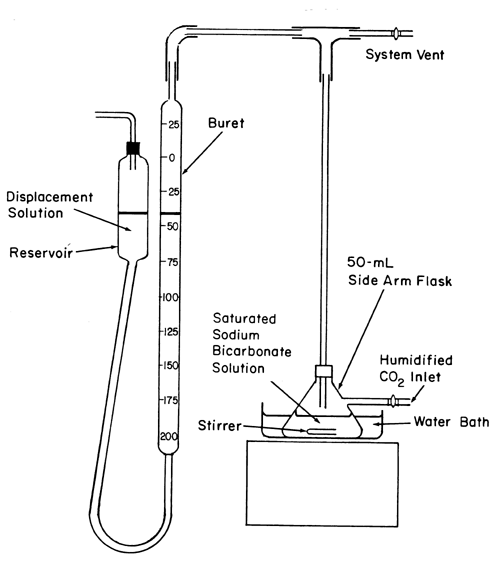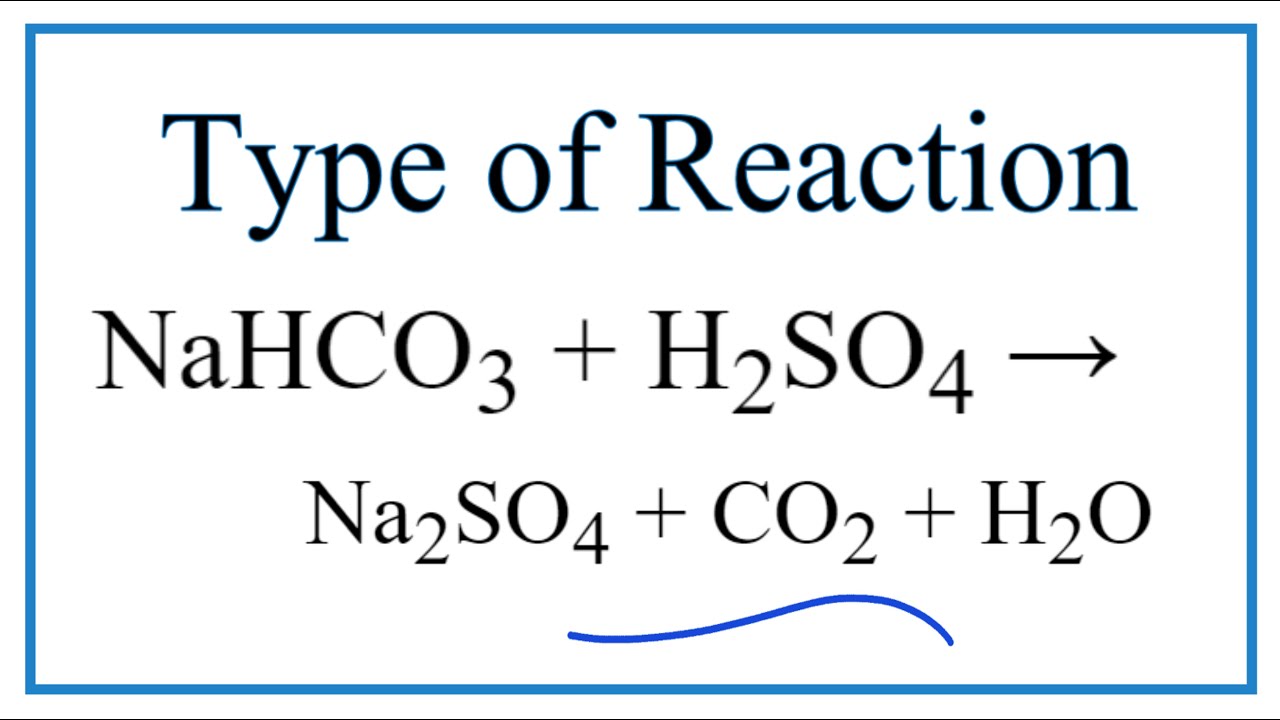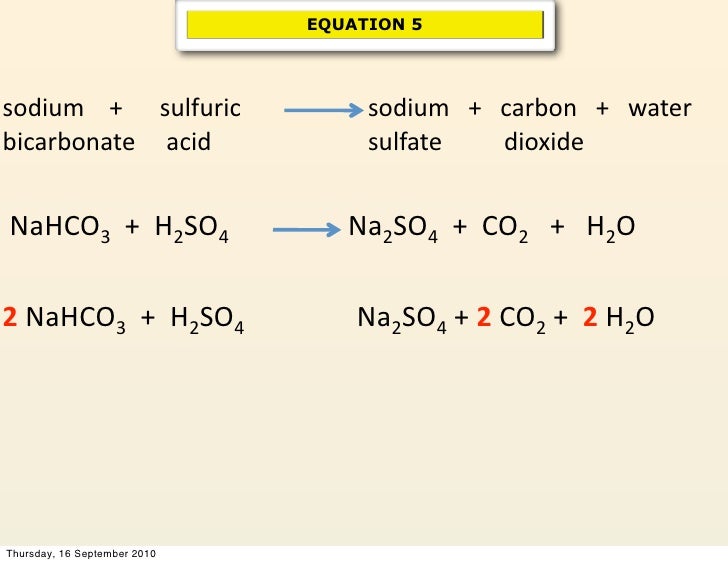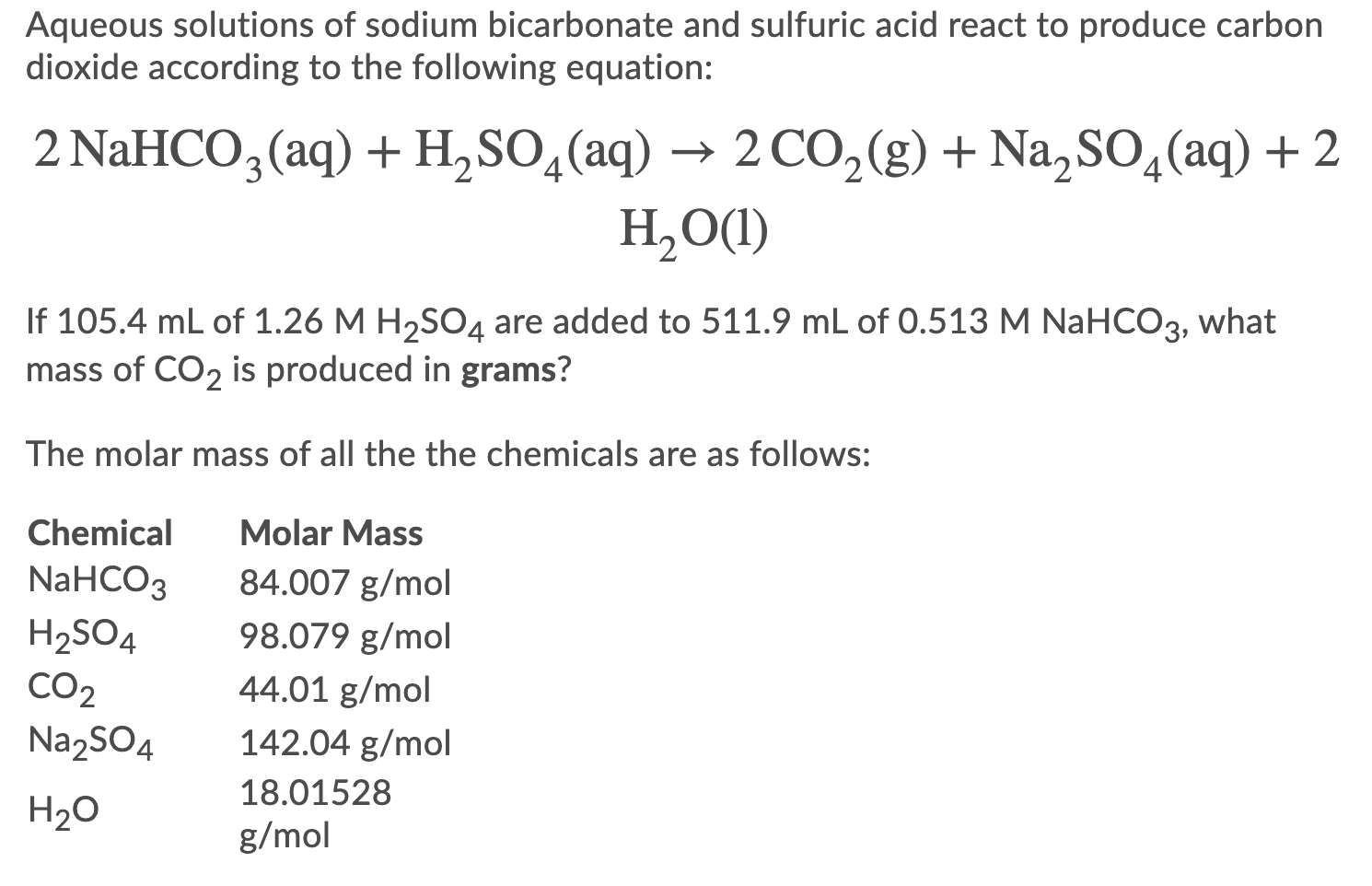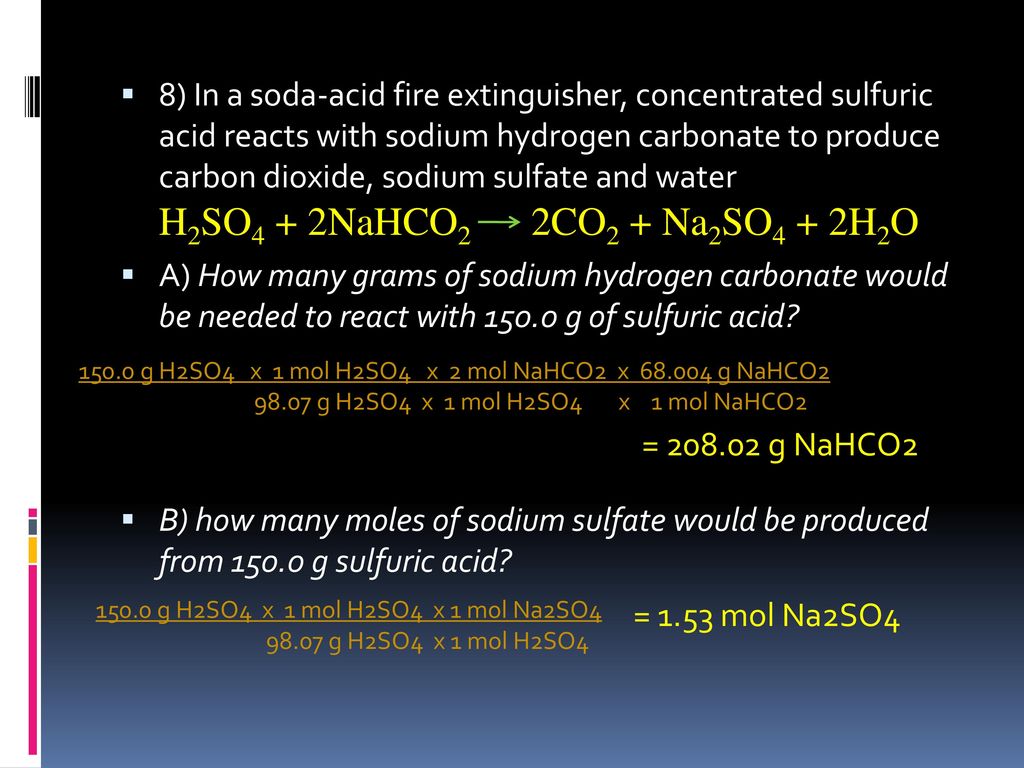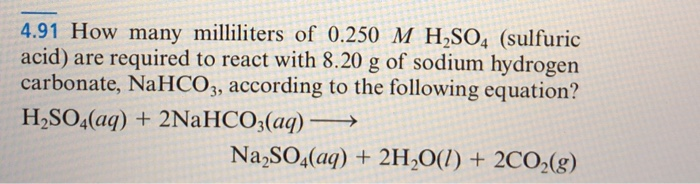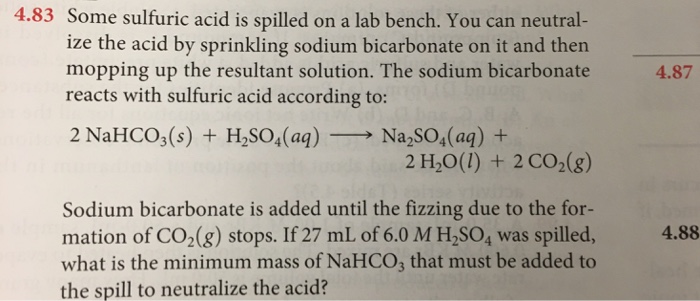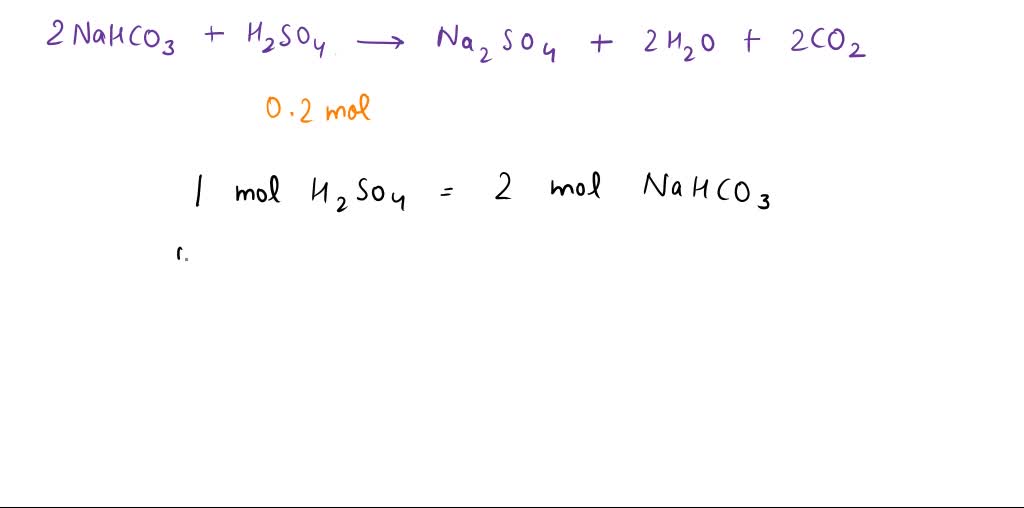
SOLVED: Sodium hydrogen carbonate (NaHCO3) reacts with sulfuric acid (H2SO4) to form sodium sulfate, carbon dioxide and water. What is the mass of sodium hydrogen carbonate required to neutralize 0.200 moles of

A student dissolves 3g of impure potassium hydroxide in water and makes the solution up to 250cm3. The student then takes 25.0cm3 of this solution and. - ppt download

What are Acids? An acid is any compound that yields hydrogen ions (H + ) or hydronium ions (H 3 O + ) when dissolved in water. Hydronium ions are really. - ppt download
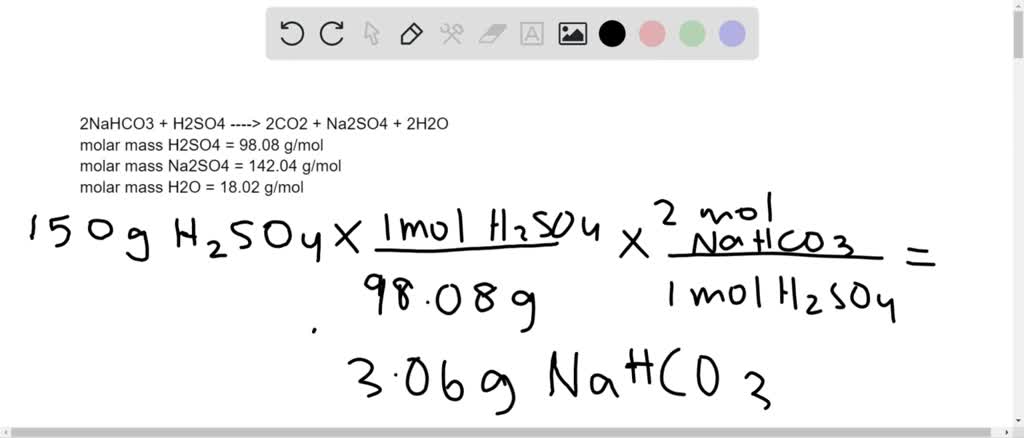
SOLVED:In a soda-acid fire extinguisher, concentrated sulfuric acid reacts with sodium hydrogen carbonate to produce carbon dioxide, sodium sulfate, and water. a. How many moles of sodium hydrogen carbonate would be needed
Sodium carbonate reacts with dil. H2SO4 to give the respective salt, water and carbon dioxide. - Sarthaks eConnect | Largest Online Education Community

NaHCO3+H2SO4=Na2SO4+H2O+CO2 balance the equation @mydocumentary838. nahco3+ h2so4=na2so4+h2o+co2 - YouTube

balance the equation Sodium bicarbonate + Sulphuric acid = Sodium sulphate + Water + Carbon dioxide. - Brainly.in

Write the balanced ionic equation for the reaction of sodium bicarbonate with sulphuric acid - YouTube